
- Articles
The neurobiology of learning and memory — as related in the memoirs of Eric R. Kandel by Miguel A. Faria, MD
This magnificent tome by Eric R. Kandel, M.D., a psychoanalyst and neuroscience researcher, is both a delightful autobiography and a scrupulously detailed history of the neurobiology of learning and memory, a relatively new area of neuroscience that Kandel refers to as a “new science of mind.”[7] His own fundamental work in this area made him a recipient of a Nobel Prize in Physiology or Medicine in 2000, which he shared with two other distinguished investigators, Drs. Arvid Carlsson (for elucidating the effects of dopamine in Parkinson’s disease) and Paul Greengard (for the discovery of signal transduction in neurons).
ESCAPING FROM AUSTRIA AND THE NAZIS
From the outset in the first chapter of his book, Kandel reminds us that the study of memory is fascinating and tells us that “remembering the past is a form of mental time travel.” Indeed it is. Kandel was born in Vienna, Austria, but left the country as a child (age 9) with his family in 1938 to escape the Nazi juggernaut on the heels of Germany’s Anschluss with Austria (March 12, 1938). The Kandels were the fortunate ones — a Jewish family that managed to escape together before the Holocaust and reach safety and freedom in America. Sadly, Kandel witnessed the enthusiastic reception by which the annexation of Austria was accomplished, and this left him with a disturbing impression as a child, poignant memories he never forgot, but that did not squelch his love for Vienna as an artistic and cosmopolitan city, where music and the arts had flourished to a pinnacle in European civilization. The Kandels might have lost their home and possessions, but they maintained a lust for life, the arts, and learning. Kandel’s nostalgia for his old country Austria and urbane Vienna, is palpable in his writing. His love for science in the footsteps of the humanities (his first love) never left him, but only grew in the United States. And so, Austria’s loss was to be America’s gain. However, it was by chance that Kandel dedicated his life to medical science, particularly research in neurobiology. Throughout his life, Kandel and his wife Denise imbibed the arts and had a cosmopolitan taste for music and opera.
Kandel’s personal experiences are inextricably entwined with learning and research in neuroscience. It is not surprising that in addition to writing books and textbooks on the subject, he also chose to write his autobiography as a vehicle to inform us about his search for the neurobiology of memory, along the way helping develop what he calls the “science of mind,” establishing the neural, physiological, biochemical, and molecular basis for the clinical specialization of neurology and neuropsychiatry.
Kandel scrupulously gives credit where credit is due in his detailed history of the science of mind as it pertains directly to his subject matter — the neurobiology of learning and memory. With so much credit spread around at times, we wonder how he was chosen for the Nobel Prize from the myriad of colleagues who contributed so many pieces to the puzzle of memory function. Indeed, several other colleagues with whom he worked for over half a century also received Nobel Prizes in Physiology or Medicine or in Chemistry. So Kandel worked in a firmament of stars. He shared his Nobel Prize with two other scientists he knew well. Given the voluminous amount of material in this tome, it is necessary to provide an extended summary — not only of Kandel’s own seminal research in neurobiology but also recount the works of others in whom he relied for his contribution — to bring the reader up to date on what we know about the neurobiology of learning and memory.
Kandel praises American education in New York in the 1940s and has fond memories of the Hebrew day school, Yeshivah of Flatbush, which “offered secular classes in English and religious study in Hebrew, both on a highly demanding level” (p. 34). Thus, this gifted refugee from Austria learned and became fluent in Hebrew as well as English.
After a brief affair with the humanities, European history, and German literature, Kandel’s interest switched to psychiatry and neuroscience while he was still at Harvard University. He entered medical school at New York University in 1951 with the intention of becoming a psychoanalyst. Kandel immersed himself in the study of Sigmund Freud’s psychoanalytic theories, and hoped to learn the biological basis for psychiatry as well as neurology. This path took him to neuroscience research, which he conducted both as a medical student and as a resident in psychiatry. However, before we can discuss Kandel’s specific contributions, we need to do as he did in his book and review all of the historical developments that led to the zenith of his accomplishments. Much of this material would serve as an extended review or refresher course to many neurologists and neurosurgeons reading Surgical Neurology International (SNI). But for others, particularly young investigators, it may serve as a starting point for study and research in the area of learning and memory in neurobiology.
THE GIANTS IN NEUROSCIENCE WHO PAVED THE WAY
On the one hand, the discovery of classical conditioning, habituation, and sensitization in learning by the physiologist Ivan Pavlov (1894–1936; Nobel Prize winner in Physiology or Medicine, 1904) and B.F. Skinner’s school of behaviorism provided an initial impetus for Kandel’s research. On the other hand, Kandel had also been exposed to clinical psychiatry, the theory of the unconscious, psychological determinism, and other aspects of psychoanalysis from close family friends and later in his training. How he would reconcile the two areas of interest would occupy his thoughts for several years during medical school and psychiatric training.
After a brief introduction to behavioral psychology, we enter the realm of neurobiology. Here Kandel introduces the greatest neuroscientist of all times, Santiago Ramón y Cajal (1852–1934; Nobel Prize in Physiology or Medicine, 1906), the Spanish neuroanatomist, who armed with his elegant silver staining method borrowed from his technically gifted professional rival Camillo Golgi, discovered the neuron and its central role in the human brain. Cajal took neuroanatomy and neurophysiology to a zenith, deducing his Four Principles of the Neuronal Organization as follows:
1. In the nerve cell, the neuron is the fundamental unit both in the structure and function of the nervous system — the neuron doctrine.
2. Neurons connect with one another with their special processes, from axons of one cell to the dendrites of another. Brain cells have a presynaptic terminal in the axon, and through the synaptic cleft, or synapse, connects to the postsynaptic site of the dendrite of another.
3. Neurons have specific connections with one another — maintaining connection specificity.
4. Neural messages travel in only one direction — i.e., unidirectional transmission — referred to as dynamic polarization (p. 64-66).
In time, action potentials in the neuronal membrane, the chemical theory of synaptic transmission through neurotransmitters between neurons, would be added to Cajal’s Four Principles through the contribution of other gifted investigators who followed in his wake. Kandel, therefore, stood on the shoulders of giants. The following background material is paraphrased, summarized, or extracted from Kandel’s book, pages 71-92, as noted in the text:
Charles Sherrington (1857–1952) studied the mechanism of the neural reflex and found that neurons could be inhibited as well as excited, and the integration (summation) of signals determines the final action. Inhibitory neurons establish reciprocal control (p. 71).
Edgar Lord Adrian (1889–1977) used a metal wire to record electrical signals in a single neuron, the action potentials that he actually could hear on a loudspeaker. The action potential is a constant, all-or-none signal. Once generated, the action potential travels at the same amplitude and at the same speed along the length of the axon. The intensity of the action depends on the frequency with which the action potential is generated. The nature of the action or thought conveyed depends on the neuronal circuit activated, as Cajal had predicted. Sherrington and Adrian, good friends, shared the Nobel Prize in Physiology or Medicine in 1932 for their contributions to discovering the function of neurons, and confirming and extending Cajal’s neuron doctrine.
The next step was taken by Julius Bernstein (1902), who followed up on the electrophysiological studies of Herman von Helmholtz, and found that the neural cell membrane had a resting potential, a difference in voltage between the inside of the cell and the environment outside. He found this voltage to be 70 mV, with the inside of the cell having greater negativity than the outside (p. 80). Inside of the cell (cytoplasm), high concentration of negatively charged proteins is balanced by positive potassium ions. The membrane is selectively permeable, through ion channels, only to potassium (K+) ions, which flow along a concentration gradient from within the cytoplasm to the extracellular fluid outside the membrane. This balance of ions maintains the stable membrane potential at −70 mV. When an action potential is generated, the selective permeability temporarily breaks down permitting ions to enter or exit the cell.
Next came the Ionic Hypothesis, a discovery shared by Alan Hodgkin (1914–1998) and Andrew Huxley (b. 1917), who in 1934 “studied the action potential generated in the giant axon of the squid.” The action potential was 110 millivolts with the upstroke high depending on sodium ions (Na+) in the extracellular fluid and the downstroke reflecting the K+ concentration. Ion channels were selectively permeable to Na during the upstroke of the action potential. These Na and K channels opening and closing in response to stimuli were referred to as voltage-gated channels. The K channels that Bernstein had previously discovered responsible for resting membrane potentials were called non-gated potassium channels. A stimulus reduces the cell’s resting membrane potential, and if strong enough, it causes the normally closed voltage-gated Na channel to open, Na rushes into the cell, creating a momentary surge in positive charge — the membrane potential is altered from −70 mV to +40 mV. After a split second, the voltage-gated K+ channel opens briefly with outflow of K+ producing the downstroke of the action potential, and returning the resting membrane potential to its original voltage of −70 mV.
For their formulation of the ionic hypothesis and the discovery of how electrical signals are generated within neurons, Hodgkin and Huxley shared the Nobel Prize in Physiology or Medicine in 1963. Later molecular biology disclosed that the voltage-gated sodium and potassium channels were proteins (p. 81-90).
The chemical theory of synaptic transmission arose from the finding that action potentials reaching the terminal axons of neurons of the autonomic nervous system (ANS) released neurotransmitters into the synaptic cleft. This was the work in the 1920s and 1930s of Henry Dale, a British pharmacologist, and Otto Loewi, a German physiologist. They found that at the end of the vagus nerve, the neurotransmitter released was acetylcholine, and that it binds to a receptor causing the heart to slow down. Conversely, the heart could speed up by the release of adrenaline. The acetylcholine receptor was later found to be a protein that also had an ion channel component that opens when acetylcholine binds to the receptor. Ion channels gated by chemical transmitters respond only to specific neurotransmitters, and they allow both Na+ and K+ ions to flow through. Action potentials from neuron to muscle fiber cause it to contract. For demonstrating that chemical signals (neurotransmitters) “went across synapses from one neuron to another in the nervous system, Loewi and Dale shared the Nobel Prize in Physiology or Medicine in 1936” (p. 91-92).
The essential task of neurons then, as predicted by Cajal, is the integration of neural activity — the actions taken by the postsynaptic neurons from the summation of excitatory and inhibitory synaptic potentials impacting on it as received from the presynaptic neurons. Action potentials occur when overwhelming excitatory signals reach a critical threshold. The most complex motor activity thought, emotions, etc., take place as a result of this integration of neural activity.
The mechanism of chemical synaptic transmission, in turn, was largely the contribution of three scientists — Stephen Kuffler (1918–1980), who studied the dendrites of cray fish; John Eccles (1903–1997), who confirmed synaptic inhibition and excitation produced by specific neurotransmitters in the spinal cord; and Bernard Katz (1911–2002), who described the mechanism of synaptic excitation and chemical transmission from motor neurons caused by electrical signals on muscle cells or other neurons. Disease states occur with neurotransmitter dysfunction. In myasthenia gravis, for example, antibodies destroy acetylcholine receptors in muscle cells resulting in autoimmune disease of generalized muscle weakness. Kandel continued:
The main excitatory neurotransmitter in the central nervous system (CNS) is the amino acid glutamate, while the major inhibitory transmitter, also an amino acid, is GABA (Gamma aminobutyric acid). Katz went on to find that in the presynaptic terminal of the signaling cell, voltage-gated calcium channels open as a result of action potentials, causing the transformation of the electrical signal of the action potential into a chemical signal. Conversely, in the receiving cell, transmitter-gated channels translate chemical signals in the synapse into electrical signals in the cell (p. 100). Neurotransmitters are released in discrete packets of 5000 molecules — i.e., quanta — from the synaptic vesicles. Flow of calcium then causes the synaptic vesicles to fuse into the cell membrane of the presynaptic terminal, opening a pore through which the transmitter is released in the synaptic cleft (p. 101-102).
Kandel came to believe that all mental functions, including the workings of the mind, are biological rather than immaterial, opposing the dualism that had held sway in some academic circles since the time of René Descartes (1592–1650). The Cartesian philosophy supported the dual nature of man: the body being material and the mind or soul is immaterial and indestructible (p. 117), a view embraced by the Roman Catholic Church. Kandel rejects this and asserts the human mind can be studied with biological tools and simple experiments using the reductionist approach he used in the new “science of mind.” He further posits that eventually all mental disorders, including those categorized as “functional” (or psychological) will be found to have a structural, biochemical, and/or molecular basis, and that the old subjective criteria for psychiatric illnesses will completely give way to the new biological “science of mind.” Not all psychiatrists agree with Kandel. And at the polar opposite, the eminent psychoanalyst Thomas Szasz, M.D. (1920–2012), author of The Myth of Mental Illness (1961) and The Manufacture of Madness (1970), wrote: “I say ‘mental illnesses’ are not diseases, despite the fact that medical and legal authorities call them ‘diseases,’ that they are treated with drugs, that those receiving these drugs are called ‘patients,’ and that the professionals treating them are called ‘physicians.’ Why do I say this? Because the established scientific criterion for the disease is a derangement in the structure or function of cells, tissues, and organs — criterion mental illnesses fail to meet, as they can be neither detected nor diagnosed by examining cells, tissues, or organs. Rather, mental illnesses are identified by certain behaviors…”[10]
While I agree with Kandel that complex human behavior and even mental functions can be studied with scientific methodology, I also believe we are far from understanding the abstractions of the mind and the integration of human consciousness. We will return to this discussion later.
For Kandel, the two great anatomists — the French surgeon Paul Broca (1824–1880) and the German physician and neuropathologist Carl Wernicke (1848–1905) — had ended the issue of duality by the mid and late 19th century when they proposed that specific mental functions were assigned to specific regions of the brain. Broca’s motor aphasia and Wernicke’s sensory aphasia affect different but discrete areas of the left hemisphere. In the case of the left frontal region (Broca’s area), a lesion causes the inability to speak or write speech; damage to the left temporal region (Wernicke’s area) results in the inability to understand written or spoken speech. Moreover, dealing with language functions, Broca’s and Wernicke’s areas are connected by associated neural pathways (the arcuate fasciculus) (p. 123). Nevertheless, structural aphasias are neurological derangements that follow the definition of disease, as defined by Szasz, rather than with functional or thought disorders as defined by traditional psychiatry.
IMPLICIT AND EXPLICIT MEMORY
Kandel distinguished different types of memory, and he defined them as follows: Explicit memory is the storage of information about events, people, and places requiring conscious attention for recall and that can be described verbally. It is also referred to as declarative memory associated with the usual recollection of events in life. Implicit memory, on the other hand, is the storage of information about habits, perceptual, and motor functions that are attained without conscious awareness for recall. It is also referred to as procedural memory. We should add that this is the type of “memory” that was attained and reinforced in lower animals by Kandel and associates by stimulation of reflexive behavior, as we shall describe in the pages that follow.
The neurosurgeon Wilder Penfield (1891–1976) at the Montreal Neurological Institute elicited explicit memory by stimulation of certain areas of the brain, and another neurosurgeon William Scoville at Hartford in Connecticut found that the temporal lobe was essential for memory. Brenda Milner (b. 1918), also at Montreal, went to Hartford in 1953 to study a patient of Scoville, “patient H.M., who had undergone bilateral removal of the medial temporal lobes, including the hippocampi,” for a severe seizure disorder. The removal of the hippocampi resulted in devastating explicit memory loss. H.M. had excellent short-term memory (being able to recall it up to a few minutes) as well as working memory, a function of the prefrontal cortex. He also had long-term memory intact (p. 127). This type of memory is stored throughout the cerebral cortex at or near the areas that originally processed the information. (We now know, e.g., that visual images are stored in the occipital lobe in the visual cortex; auditory memories, in the temporal-parietal auditory cortex, etc.). The problem with H.M. was that he was unable to convert new short-term memories into new long-term memories so that he forgot events shortly after they had taken place.
Long-term implicit memory “for skills, habits, and conditioning learning are stored in the cerebellum, striatum (i.e., caudate nucleus, putamen, and perhaps the nucleus accumbens, and globus pallidus) and the amygdala” (p. 130). Drawing a star and improvement with repetition of the task (without conscious awareness or remembrance as in the case of H.M., who drew such a star for Milner) is implicit memory that requires striatum and cerebellum rather than hippocampus. Another instance of learning implicit memory is riding a bicycle, a task requiring multiple cerebral areas for long-term storage. Implicit (unconscious) and explicit (conscious) memories then require two different anatomical systems. The two are not comparable, and I suspect that while implicit memory can be studied by the reductionist approach discussed in this book, explicit memory in the human brain is a more intricate process requiring more complex methodologies.
One point that Kandel repeatedly makes is that because of evolutionary development, animals, including invertebrates, such as the sea snail (Aplysia), and the fruit fly (Drosophila), maintain the biological systems needed for survival, and this includes simple neuronal circuits involved in flight or flght reactions. Moreover, the inference is that complex (conscious) explicit memory in humans is only more complex and only quantitatively different from simple reflexive implicit memory in the sea snail. The intimation is that the puzzle of learning and memory is largely solved; all we have to do now is to work out the mechanism for human consciousness, and then once and for all, we can end the concept of the duality of mind and body and delegate Plato and Descartes to the dustbin of metaphysics! As I will discuss later, with due respect to Kandel, this is far from being the case.
Another dilemma that is purportedly solved by evolutionary theory is the associated phenomenon of the random process of natural selection and adaptability accounting for similar neurobiology in all animals. This implies that, for example, by studying the neural circuitry of the sea snail (Aplysia) or the earthworm (annelid) that we can learn the intricacies of the human brain. But here again, Charles Darwin (1809–1882), who once suggested that his greatest teacher was not Lamarck, Linnaeus, or Cuvier — but “old Aristotle,” may not have all the answers as far as the evolution of the human mind. Although Aristotle and Darwin both believed in a hierarchical systemization of animals, Darwin, of course, believed in natural selection as a random event in evolutionary theory. Aristotle believed in intelligent design, a teleological theory of final cause — i.e., an efficient universe in which all actions have been designed by the prime mover (God) toward an end.[4] Plant and animal life preserve systems because in following utilitarian principles, nature does not waste its resources uselessly. In this regard, Kandel’s proposition is consistent with current thinking: “In the course of evolution, humans have retained some of the cellular mechanisms of learning and memory storage found in simpler animals,” not according to him by intelligent design but by random natural selection (p. 144). It may turn out in the end that Kandel is correct, but the scientific proof is not yet in evidence.
APLYSIA AND THE REDUCTIONIST APPROACH TO LEARNING AND MEMORY
In Chapter 9, “Searching for an Ideal System to Study Memory,” Kandel writes that he employed a reductionist approach: “I was convinced that the biological basis of learning should be studied first at the level of individual cells and, moreover, that the approach was most likely to succeed if it focused on the simplest behavior of a simple animal” (p. 144). Because he had previously studied the signaling characteristics of large nerve cells with two French scientists, Kandel reminisces, “I remembered vividly the advantages of the cray fish’s sensory neuron for studying the properties of dendrites… I settled on the giant marine snail Aplysia as a suitable animal for my studies” (p. 145). The giant marine snail was called the “sea hare” by Pliny the Elder and Galen because, at rest measuring one foot in length and weighing several pounds, it resembles a rabbit. Indeed it was a suitable organism to study because Aplysia has only 20,000 brain cells that are organized in nine discrete clusters, and some of the cells are so large they can be seen and its simple connections easily studied with the naked eye, “making it relatively easy to insert microelectrodes into them to record electrical activity” (p. 147).
Kandel sought to stimulate the neurons of Aplysia, in the same manner as Pavlov’s conditioning stimuli in live animals induced learning, hoping to correlate behavior with plastic changes in the synapse of neurons (p. 159). Such changes, he posited, may result in memory storage. He called his reductionist methodology Neural Analogs of Learning because in an isolated ganglion of Aplysia, he simulated the sensory stimuli used in Pavlovian learning experiments by electrically stimulating axons ending on target cells. He also elicited Pavlov’s behavioral protocols — for example, habituation, sensitization, and classical conditioning — translating them into biological protocols (p. 160).
Kandel used three types of stimulation to single neurons in the abdominal ganglion of Aplysia’s brain. (A functionally related neural cluster in the CNS of an invertebrate animal is referred to as a ganglion) (p. 167). Habituation was effected with a weak and harmless stimulus; sensitization, with a noxious stimulus. And classical conditioning took place when a noxious stimulus was paired with a benign stimulus, and the cell responded to the benign stimulus as if it was a harmful stimulus. His Neural Analogs of Learning protocol was summarized by Kandel as follows:
- Habituation: Stimulus to a bundle of axons impinging on cell R2 of the abdominal ganglion of Aplysia. Ten weak stimuli in succession resulted in homosynaptic depression.
- Sensitization: Five strong stimuli to a different pathway leading to R2 cells resulted in heterosynaptic facilitation.
- Aversive Classical Conditioning: Weak stimulus in one pathway and a strong stimulus in another pathway of Aplysia — the pairing of the stimuli, elicited an enhanced response to the weak stimulus (p. 169-170).
The R2 cells of the abdominal ganglion of Aplysia are very large — 1 mm in diameter, visible to the naked eye so that microelectrodes are easily inserted, much easier to study than the hippocampal cells of larger animals. Kandel was able to ascertain that there were plastic changes in neural connections as a result of stimulation and learning.
The momentous idea that enhancing synaptic transmission by reinforcing neural connections might promote learning had been proposed by Cajal in 1894 (p. 158). Moreover, in fact, by 1948, the Polish neuropsychologist Jerzy Kornorski expounding on Cajal’s postulates had gone on to promote the concept of neural plasticity after actually observing that repeated stimulation of neurons resulted in functional and anatomic transformations at the synapse. In addition, Kandel’s reductionist approach linking cellular neurobiology and synaptic plasticity to learning and memory were based on his assumption learned from ethology (the study of animal behavior in their natural habitats) that “learning is preserved through evolution because it is essential for survival” (p. 186). Moreover, this assumption was extended from invertebrates and the more simple animals in evolutionary history to more advanced animals, including man. The neural mechanism of learned fear in the mollusk Aplysia, Kandel posited, could, therefore, be extended to the study of learned fear in the amygdala of genetically modified mice as well as in humans.
In 1965, Kandel arrived at New York University and founded the new Division of Neurobiology and Behavior (p. 184). In 1967, he announced the new direction in neurobiology in a major review entitled, “Cellular Neurophysiological Approaches in the Study of Learning” (p. 185), moving beyond analogs of learning to the study of synaptic plasticity in learning and memory. Again, Kandel used Aplysia — specifically the gill-withdrawal reflex mediated by the abdominal ganglion with which he was very familiar (p. 188).
Kandel explains that the gill is the external organ through which Aplysia breathes: It is protected by the mantle shelf that ends in a siphon, a spout through which the animal expels waste and seawater. “Touching the siphon produces a quick withdrawal reflex of the vital siphon and gill to protect them from damage” (p. 189). This reflex was subject to both habituation (simple touch to the siphon) and sensitization (“a strong shock to either head or tail) with a short-term memory that lasted a few minutes.”
Kandel and associates found that “long-term memory (lasting a few days) in Aplysia, as in people, requires repeated training interspersed with periods of rest. Practice makes perfect, even in snails” (p. 191). Training with rest periods for Aplysia, “ten stimuli every day for 4 days produce habituation that lasts for weeks” (p. 192).
By 1983, Kandel and associates had reliably and successfully accomplished classical conditioning for the gill-withdrawal reflex (p. 192). By 1985, after 15 years of work, they demonstrated that “simple behavior in Aplysia could be modified by learning” and that changes took place in the synaptic connections in the neuronal circuit mediating the behavior of the gill-withdrawal reflex (p. 200). Although genetics specify the types of connections between neurons, the strength and effectiveness of those connections are modified and re-enforced by learning and experience (p. 202).
After sensitization with the series of fired action potentials, connections are enhanced in both the gill-withdrawal reflex, as well as causing the ink glands of Aplysia to secrete, an additional inking response (p. 204). Habituation weakens synaptic connections, and sensitization strengthens them. Memory is stored within cellular mechanisms throughout the entire neural circuit involved in the learning response, the duration of learning-dependent on the length of time the synapse is reinforced.
A GLIMPSE AT LONG-TERM MEMORY
Learning and memory in Aplysia are effected by the mediating circuit — a homosynaptic change in the direct neural connections — and it is modified by the modulatory circuit — a heterosynaptic change by neurons in a different circuit and in a different part of the body (p. 205). Classical conditioning induces both homosynaptic and heterosynaptic changes and thus affects both mediating and modulating circuits of the learning process. “Short-term memory is converted to long-term memory” by repetition (again, practice makes perfect!). Memory becomes established (consolidation) by association with knowledge already established, and it requires a certain amount of time — two or more hours — to become fixed. Before this time, memory has not become fixed and can be disrupted, as is seen in retrograde amnesia and memory loss just before an epileptic seizure in humans (p. 211).
Long-term memory storage requires the production of new protein in the cell, and it is accompanied by new active presynaptic terminals on other neurons and with the increased synaptic transmission (p. 212). Long-term habituation causes sensory neurons to retract active terminals and markedly decrease synaptic transmission, while long-term sensitization causes neurons to grow new terminals and synaptic transmission on the motor neurons [Figure 1].
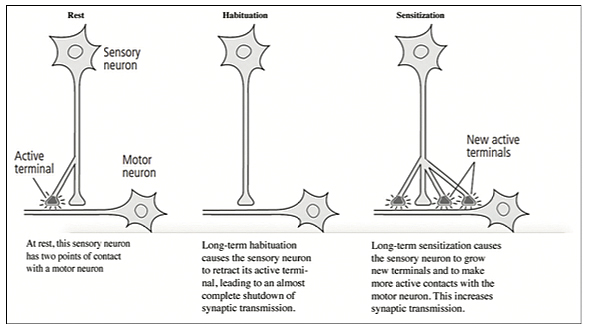
As memory declines (from the passage of time or lack of use), the number of synaptic connections drop. The number of synapses increases with learning and repeated use. “Long-term memory persists for as long as the anatomical changes are maintained” (p. 215). Short-term memory results from functional use of the synapse, but long-term memory requires anatomical changes, “To be useful, memory has to be recalled” (p. 215).
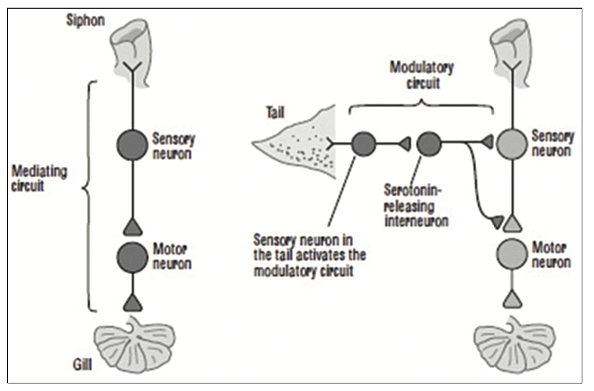
Studies demonstrate that changes in the mammalian brain also take place secondary to learning, and these changes are more readily achieved in the early years of life when the brain is more capable of undergoing plasticity and more pliable by training and practicing skills (p. 218). Plasticity is the ability of neurons and synapses to change anatomically and functionally in response to different patterns of stimulation in the laboratory or to usage in the normal life of the organism. Neurotransmitters are essential in the maintenance of connections between neurons, in conducting transmission, and in effecting plastic change. Habituation results in less, while sensitization results in more, neurotransmitter release (p. 222). A shock to Aplysia’s tail activates directly the mediating neurons for the gill-withdrawal reflex releasing glutamate, as well as a second class of neurons, modulatory interneurons, that have slow synaptic potentials and release serotonin. These serotonin-releasing interneurons are heterosynaptic and modulatory, modifying or fine-tuning the strength of the gill-withdrawal reflex. The modulatory circuit then enhances the response to the touching of the siphon, telling the snail implicitly that it must pay attention to its safety (p. 223-224) [Figure 2].
RECEPTORS AND FIRST AND SECOND MESSENGERS (CYCLIC AMP)
The neural circuitry involves biochemical and molecular signaling pathways. Cyclic AMP, the master regulator of signaling within cells, is also involved in neural circuitry. When epinephrine and other hormones reach the cell membrane, they attach and stimulate receptors at the cell surface, acting as first messengers. Epinephrine causes the release of cyclic AMP within the cell; as such, the master regulator acts as the second messenger (p. 226).
Receptors are actually specialized proteins in the postsynaptic cell that recognize specific neurotransmitters and bind them at the cell surface. Some receptors bind neurotransmitters; others serve as gated ion channels or activate second messengers. Ionotropic receptors span the cell surface with neurotransmitter binding sites having channels by which ions can pass in and out of cells. Metabotropic receptors bind neurotransmitters or hormones (first messengers) and then activate chemicals inside the cell (second messengers) that initiate a variety of cellular responses.
Cyclic AMP, the most common second messenger, is produced by the enzyme adenylyl cyclase. Once activated, it binds to key proteins that mediate a number of cellular reactions. Cyclic AMP-dependent protein kinase, or protein kinase A, modifies other proteins in the cell by adding phosphate molecules — a process called phosphorylation – which activates some and deactivates other proteins in a largely reversible process referred to as signal transformations or signal transduction in the nervous system.
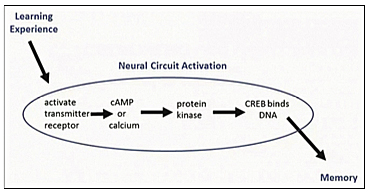
Kandel describes the biochemical steps in short-term memory as follows: “A shock to the tail of Aplysia activates an interneuron that releases the chemical messenger seratonin [first messenger] into the synapse. After crossing the synaptic cleft, serotonin binds to a receptor in the sensory neuron, leading to the production of cyclic AMP. Cyclic AMP frees the catalytic unit of protein kinase A. The catalytic unit of protein kinase A enhances the release of the neurotransmitter glutamate” (p. 229). Cyclic AMP, the second messenger, then is involved in the formation of short-term memory in Aplysia.
Calcium (Ca++) is essential for the release of glutamate, allowing it to flow into the presynaptic terminal. “Cyclic AMP and protein kinase A act directly on the release of synaptic vesicles further releasing glutamate into the synapse” (p. 231-232).
The experiments in Aplysia complemented those in the fruit fly, Drosophila, conducted at Columbia University where the simple genetic makeup and short reproductive cycle made these lower animals amenable for studies in genetics and learning. Because survival mechanisms, including biochemical pathways and molecular biology, have been conserved through evolution, Kandel reminds us, “evolution does not require new, specialized molecules to produce a new adaptive mechanism” — the cyclic AMP pathway is not only used by such organisms as Drosophila and Aplysia but also man. Moreover, cyclic AMP is not only unique to memory storage but also used in a variety of other specialized cellular functions. Likewise cellular mechanisms used in implicit memory are posited to be similar in many organisms from insects and invertebrates to man (p. 234).
Evolution uses the same genes in slightly different ways in different organisms, coding for slightly different proteins — “but new capabilities are achieved by modifying existing molecules” (p. 236). “Few proteins are unique to the human brain,” writes Kandel. All life uses the same biological building blocks. Short-term memory results from the strengthening of synaptic connections in sensory and motor neurons using cyclic AMP, protein kinase A, and the release of glutamate at the presynaptic terminal.
THE JACOB-MONOD MODEL OF GENE REGULATION AND THE FORMATION OF MEMORY
A key advance in molecular neurobiology was the development of recombinant DNA and gene cloning, which gave impetus to the biotechnology industry. One of the major breakthroughs was the use of recombinant DNA technology in the creation of unlimited amounts of human insulin (p. 248).
In 1974, Kandel replaced his mentor Harry Grundfest at Columbia University and became affiliated with the Howard Hughes Medical Institute as a senior investigator for the neural science initiative. He then proceeded with the next phase of his research, the elucidation of the mechanism for the transformation of short-term into long-term memory. He proceeded under the following protocol:
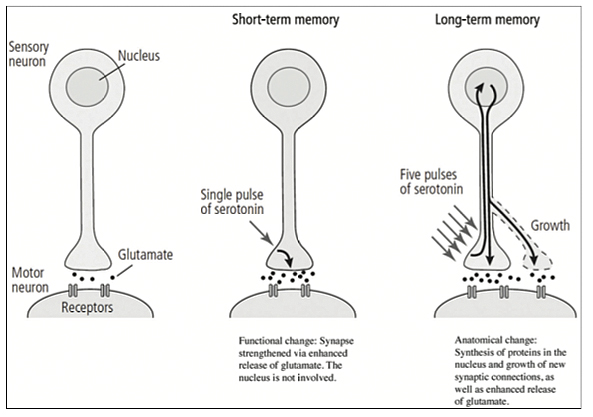
One brief pulse of the modulatory neurotransmitter serotonin was used “to strengthen the synaptic connection between the sensory and motor neurons for a few minutes by enhancing the release of glutamate from the sensory cell.”
“Five separate pulses of serotonin were used to simulate five shocks to the tail of Aplysia by stimulating the serotonin-releasing modulatory interneurons.” This was found to strengthen synaptic connections for days and led to the growth of new synaptic connections, an anatomical change that involved the synthesis of new protein as well as the enhanced release of glutamate (p. 256) [Figure 3].
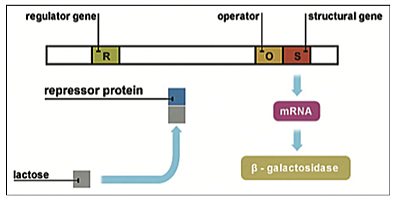
At this point we should retrace, as Kandel did, the discovery by François Jacob (1920–2013) and Jacques Monod (1910–1976) at the Institute Pasteur of the “Genetic Regulatory Mechanism in the Synthesis of Protein” (1961; Nobel Prize in Physiology or Medicine Winners, 1965) working on the Escherichia coli bacteria. The Jacob-Monod (operon) model of gene regulation proposed that genes could be regulated, switched on and off, by regulatory proteins (p. 256). Jacob and Monod postulated that effector genes encode for proteins and enzymes that mediate specific cell functions and are regulated by other regulatory genes. Regulatory genes, in turn, encode for regulatory proteins that switch those effector genes on or off by binding at the effector site. Moreover, repressor genes were found that code for the protein that shuts genes off, and activator genes that encode for the protein that turns genes on. These operon genes are turned on and off by other genes and proteins based on environmental cues and needs [Figure 4].
In 1985, bolstered by this knowledge, Kandel turned to the investigation of proteins that regulate gene expression and long-term memory, as in the Jacob-Monod model. Kandel and associates found:
Repeated pulses of serotonin produce higher concentrations of cyclic AMP causing protein kinase A and MAP kinase (associated with synaptic growth) to move into the nucleus from the cytoplasm to activate genes. Once in the nucleus, these kinase enzymes activate a regulatory protein called CREB (cyclic AMP response element-binding protein), which binds to a promoter gene. By 1990, CREB had been found in the sensory neurons of Aplysia and proved to be essential for the long-term strengthening of synaptic connections underlying sensitization.” Blocking the action of CREB in the laboratory prevented the formation of long-term synaptic change. CREB phosphorylated by protein kinase A in the nucleus of sensory neuron turned on the genes that produce long-term facilitation of synaptic connection involved in long-term memory [Figure 5].
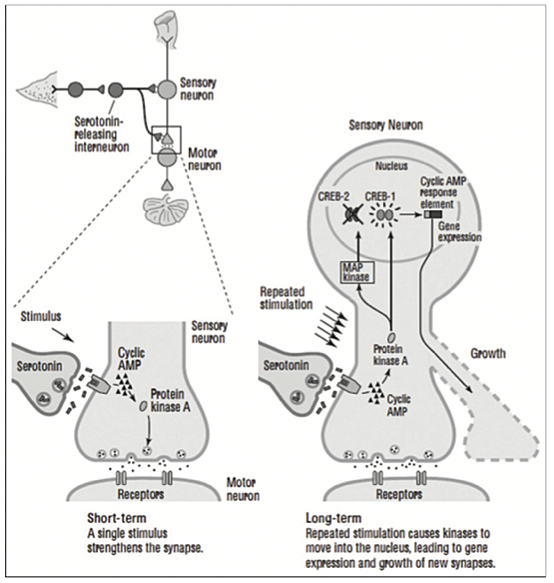
By 1995, it had been found there were two forms of CREB protein that act as predicted in the Jacob-Monod model — “one that activates gene expression (CREB-1) and one that suppresses gene expression, a repressor protein (CREB-2). Long-term facilitation of synaptic connections requires not only switching on of some genes but also the turning off of others” (p. 263). Two facts struck Kandel: That the Jacob-Monod model applied to memory storage and that Charles Sherrington’s integration of neural action played dual roles — that is, that “excitatory and inhibitory synaptic signals converge on a neuron” so that the two CREB regulators integrate opposing signals (p. 265).
At this point, Kandel hypothesized the mechanisms underlying flashbulb memories, exceptional good memories, the age-related memory loss of “benign senescent forgetfulness,” etc. All of these phenomena, he believes, may be associated with relative activity or deficiencies of CREB-1 and CREB-2. For example, benign senescent forgetfulness may be due to deficiency of CREB-1 and an excess of CREB-2 (p. 266). Similar CREB systems have been found in the mechanism of implicit memory in Drosophila, mice, and other species of animals besides Aplysia. The switch for converting short-term memory to long-term memory is similar in a variety of simple animals. Long-lasting synaptic changes require nuclear activity, gene transcription for the production of new proteins both in the cell body of the neuron and locally at the synapse.
Later, it was found that proteins synthesized locally at the synapse are required to sustain growth initiated by gene expression farther away at the cell body of the sensory neuron. Messenger RNA sent from the nucleus is activated by a cytoplasmic polyadenylation element-binding protein (CPEB) and is the vehicle for the production of local proteins. “Activated messenger RNA regulates protein synthesis at the synaptic terminal, stabilizes the synapse and perpetuates memory.” CPEB has prion-like properties. Prions (proteinaceous infectious agents) in their recessive forms are inactive, but in the dominant phase is self-perpetuating and usually toxic to nerve cells causing degenerative diseases, such as mad cow disease and Jakob-Creutzfeldt disease. However, in this instance, they acted benignly as self-perpetuating local proteins, synthesizing, and perpetuating synaptic facilitation and the storage of memory. Once the memory is established in neural circuits, it can be recalled and consciously controlled (p. 273-275).
Implicit memory cannot be recalled consciously and is responsible for motor skills, such as riding a bicycle, and playing tennis, as well as the type of memory that Kandel postulated was similar to the one he was studying with the reflexes of Aplysia. Explicit memory is more sophisticated complex memory that can be consciously recalled and expressed in images, writings or words, depending for its formation on the neural circuitry of the hippocampus and the medial temporal lobe (p. 279-281). What the brain stores is core memory but recollection of memory is a creative process in which past events can be elaborated on, reconstructed, or even distorted in various parts of the cerebral cortex. Can this complex explicit memory mechanism of the human brain be equated to the study of the simple reflex circuit involved in the learning process and implicit memory of Aplysia? I continue to have some doubts.
In 1989 at age 60, Kandel returned to the study of the hippocampus and explicit memory. The study was made easier by the discovery of the pyramidal cells of the hippocampus, the phenomenon of long-term potentiation, and the development of genetically modified mice with new research methodologies. Pyramidal cells were involved in tasks dealing with spatial maps — internal representations of the external environment formed by a combination of many specialized pyramidal “place” cells found in the hippocampus. Long-term potentiation refers to the response of hippocampal cells (from slices of hippocampus kept in special preparations in the laboratory) to bursts of electrical stimulation, 100 impulses per second, resulting in synaptic facilitation. Kandel asserted that long-term potentiation is analogous to long-term facilitation in Aplysia and that it strengthens synaptic connections (p. 283).
Glutamate is the major excitatory neurotransmitter in vertebrates and invertebrates, acting on ionotropic receptors in the hippocampus — the AMPA and NMDA receptors. AMPA receptors respond to individual action potentials in the postsynaptic neurons, mediating normal synaptic transmission. NMDA receptor responds only to bursts of stimuli and is a prerequisite for long-term potentiation. Kandel found that depolarization, generated by AMPA receptor produced by repeated stimulation of the postsynaptic neuron, causes the “ion channels in the NMDA receptor to open, allowing calcium to flow into the cell (p. 284). Calcium ions in the postsynaptic cell act as a second messenger (such as cyclic AMP), triggering long-term potentiation… Calcium activates a kinase (calmodulin-dependent protein kinase) that increases synaptic strength for about one hour” (p. 284). A good question to ask here is: Do these methodologies consisting of in vitro high-frequency stimulation in the lower animals apply to in vivo normal transmissions in higher animals and man?
This synaptic strengthening mediated by calcium influx only takes place when the presynaptic cell is active and “releasing glutamate and the AMPA receptor in the postsynaptic cell binds glutamate and causes cell depolarization” (p. 284-285). The two events must occur for long-term potentiation to take place. Nevertheless, as Kandel admitted, the studies were highly artificial methods to produce synaptic strength.
In fact, selective breeding of genetically modified mice and recombinant DNA technology proved to be a more appropriate methodology to study synaptic strength and memory storage (p. 287). Selective breeding was helpful in isolating particular behaviors in mice, and genetic defects were correlated with a mouse spatial (hippocampal) memory in the laboratory (p. 291).
In mice, the predominant neurotransmitter is dopamine, which in the mammalian brain is released at the synapse for attention and reinforcement (p. 293). Dopamine activates an enzyme that increases cyclic AMP, which in turn recruits protein kinase A and other protein kinases, which usher in CREB-1 and the switching on of the effector genes. Likewise, blocking CREB-2 in Aplysia enhances the strength and increases the number of synapses, similarly as in facilitating long-term potentiation in the hippocampus and forming spatial memory in the mouse. Spatial memory is explicit memory for finding one’s way around in external space. Unlike the simple reflex action of implicit memory of Aplysia and Drosophila [Figure 6], the more complex explicit memory in the mammalian brain would require several gene regulators in addition to CREB for neural circuit activation (p. 294-295). Moreover, the encoding, storage, and recall of spatial and other forms of explicit memory in the mammalian brain require conscious attention (p. 295).
SPATIAL MEMORY AND CONSCIOUS EXPERIENCE
Cognitive and Gestalt psychologists believed in the “Kantian notion that the brain is born with a priori knowledge built-in, innate knowledge independent of learning and experience.” They also upheld the notion that the brain reconstructs perception from experience as well as from built-in neural circuitry and that the brain is capable of doing this by “developing an internal representation of the external world — a cognitive map that is ultimately used to carry out purposeful action” (p. 298). This turned out to be true in the case of the mapping of the somatosensory cortex and the work of Wade Marshall in the cat and Wilder Penfield in the human brain. But is this also true for complex psychological interactions, thinking, and the nature of attention, cognition, conscious, and unconscious processes of the human brain?
The binding problem of unconsciously integrating multiple sensory modalities carried separately in different cerebral pathways and reforming them into a cohesive whole is a problem unresolved in neurobiology. Learning, integration into memory, and unconscious information processing make sense of the external world with or without the abstract notion of internal representation. Spatial representation of the well-known homunculus of the somatosensory cortex and the integration of the visual system in the parieto-occipital area in the mammalian brain are a far cry from the internal spatial representation of the outside world in the human brain and its association with complex learning, explicit memory, and consciousness. Cognition and the maintenance of continuous awareness of our surroundings are much more complex functions that defy present tools in neurobiology and Kandel’s “science of mind.”
Unlike the primary motor, somatosensory, or visual cortices, “the hippocampus is concerned with spatial perception of the environment and represents multisensory experience,” and multisensory representation of space in the external world. This spatial representation is built-in the Kantian manner in mind; by this Kandel refers to the empirical concept of Immanuel Kant (1724–1824) that a priori knowledge exists at birth, although it can be modified by learning and experience as postulated by the empiricism of the philosopher John Locke (1632–1704) asserting that the human mind is a blank slate, a tabula rasa, to be filled in by learning and experience. The map of the external world is fixed by learning over the long term, and it is recalled every time it enters the same space. As Kandel likes to emphasize, practice makes perfect (p. 308-309).
The task of spatial memory in the mouse is accomplished with a “large, white, well-illuminated circular box surrounded by a rim of 40 holes,” one of which provides an escape hatch for the mouse (p. 290-291). Smart, genetically modified mice find the escape hatch using a spatial strategy that requires learning and memory mediated by the hippocampus. The wall has distinctive markings that the mouse learns to recognize as being aligned with the escape hatch. Mice in which the protein necessary for long-term potentiation has been blocked, and those in which this specific genetic defect has been engineered, were deficient in finding the escape hatch, a defect correlated with the mouse spatial memory.
Unlike implicit memory, attention must be paid for the formation of explicit learning, and navigational representation (a neural correlate of explicit memory of the external world) can be prevented from developing and being retained as spatial memory by blocking protein synthesis (p. 310). It is worth repeating that in addition to the capability of the neural circuitry to provide for protein synthesis and gene activation — selective attention is essential for perception, conscious action, and memory formation and retention. Moreover, all of these functions are necessary for the formulation and evaluation of conscious experience (p. 311). Attention is also necessary for the long-term stability of spatial maps of the external world, and it is the dopaminergic pathway extending from the midbrain to the hippocampus and prefrontal cortex that is involved in selective attention. Voluntary explicit attention is initiated by the cerebral cortex, but implicit attention can be obtained in laboratory animals with noxious stimuli, such as an electrical shock.
Serotonin is the neurotransmitter that triggers protein kinase A in the abdominal ganglion of Aplysia for the gill-withdrawal reflex, but dopamine triggers protein kinase A in the mouse hippocampus for the escape hatch (p. 314). Spatial memory in men (who rely on an internalized geometric map of distances) and women (who use familiar cues, such as houses and landmarks), as most of us have noted in giving travel directions, use different areas of the brain — for example, “the left hippocampus in men, and the right parietal and right prefrontal cortex in women” (p. 316).
FEAR, ANXIETY, AND OTHER MEDICAL DISORDERS
Serotonin deficiency has been associated with depression, and many anti-depressant drugs work by increasing serotonin levels in the brain. Dopamine deficiency has been associated with Parkinson’s disease. Excess of norepinephrine and dopamine has also been associated with schizophrenia. “Benign senescent forgetfulness in which the elderly cannot readily convert short-term memory into long-term memory has been found to be associated with loss of the synapses that release dopamine in the hippocampus as we age.” Likewise, aged mice also have difficulty with explicit spatial memory in the maze task because of dopamine neurotransmitter deficiency in the hippocampus. Drugs that activate dopamine receptors in mice reverse hippocampal-dependent memory deficit but not so in humans. The drug rolipram that blocks the enzyme that breaks down cyclic AMP also improves learning in old mice but not in humans. In Alzheimer’s disease in which deposition of B-amyloid plaques occurs in the human hippocampus, some of the damage seen in the early stages of the disease rolipram has been found “to act favorably” but no clinical improvement is noted. The same is true in benign senescent forgetfulness, and treatment with rolipram yields no clinical improvement (p. 331). What are the best learning and memory enhancers in humans? Kandel tells us, “Studying is the best cognitive enhancer for those capable of learning” (p. 333). Attention is essential, and repetition, recall, and practice make perfect. This time I could not agree with Kandel more!
Normal anxiety exists in two forms — “instructive anxiety (instinctive or innate fear) built genetically into the organism for survival and learned anxiety (learned fear), to which an organism may be genetically predisposed, but it’s still learned by experience.” These instructive and learned fears are conserved because of their survival value in animals. Fear has a conscious and unconscious component. The amygdala coordinates conscious experience and its central to both components. Learned fear initiated through sound is mediated by a direct pathway from the cochlea to the amygdala. The indirect pathway goes through the auditory cortex and then to the amygdala. Direct pathway for pain travels through the thalamus then to the amygdala. However, autonomic responses connect directly to the amygdala bypassing the cerebral cortex, perhaps preceding conscious cortical evaluation of fear.
Adaptive responses to fear that are coordinated and mobilized by the amygdala, involve the hypothalamus for visceral responses and the cingulate cortex for conscious evaluation, which clinically has been associated with the limbic system, which for some unstated reason is not even named as such or referred to by Kandel. Yet the limbic system and its connections continue to be useful to neurosurgeons, neurologists, neuropsychiatrists, as well as research neuroscientists involved in clinical medicine.[6,9] I personally continue to find useful the interconnections of Papez circuit and the limbic system to the cerebral cortex in the evaluation of fear, rage, human aggression, and their autonomic and emotional components.[5] Moreover, of course, the limbic interconnections are indispensable for neurosurgeons performing deep brain stimulation for various conditions affecting mind, mood, and the conscious state, including major depressive disorders, “obsessive-compulsive disorders (OCD), posttraumatic stress disorders (PTSD), eating disorders, as well as Parkinson’s disease.”[8]
Although Kandel does not discuss Papez circuit — the initial contribution to the concept of the limbic system — this circuit is believed to deal with the emotional components of memory. Described in 1937 by James Papez, an American physician and neuroanatomist, the circuit begins in the hippocampal formation, receives input from the amygdala, and connects with the mammillary body through the fornix. The mammillary body, in turn, sends important connections to the anterior thalamus through the mammillothalamic tract. The anterior thalamus sends strong projections to the cingulate gyrus, which, in turn, completes the circuit by projecting fibers to the parahippocampal gyrus through the isthmus in the medial region of the cerebral hemisphere. The amygdala and the hippocampus have reciprocal connections with both the hypothalamus and the cerebral cortex. The amygdala is important in motivational, social, and fear responses to the external environment, while the hippocampus, as we have seen, is important in spatial and other forms of memory. The cerebral cortex processes and integrates this information and gives it conscious awareness.
The hypothalamus mediates the ANS and the endocrine system, as well as the physiologic expressions of fear associated with the all-important flight or fight response; the cingulate cortex mediates the conscious evaluation of fear (p. 345). The benzodiazepine drugs, such as diazepam (Valium) and lorazepam, act as anxiolytic agents by dampening neuronal transmission — either binding directly to the neurotransmitter GABA receptor site or indirectly by enhancing the inhibitory effects of GABA on neuronal transmission. (The negatively charged chloride ions mediate inhibition of neurons by GABA.) Valium is also an excellent anticonvulsant presumably acting by similar mechanisms.
Studies in the amygdala have been found to yield comparable results as those of long-term potentiation in the hippocampus and long-term facilitation and learned fear in Aplysia, including the release of first and second messengers, the activation of cyclic AMP and protein kinase A, and induction of the process of the regulatory gene and protein CREB (p. 346).
“When an animal learns to associate a tone with safety, the response in the striatum (pleasure center and area of the brain involved in positive reinforcement and known to be affected by cocaine) is dramatically enhanced, consistent with pleasure (p. 349)”. The striatum consists of caudate nucleus and putamen (dorsal striatum), and the nucleus accumbens (ventral striatum); the corpus striatum includes the globus pallidus.
The prefrontal cortex, which is essential for working memory, critical judgment, executive action, and long-term planning, is detrimentally a ected by schizophrenia (p. 354). Monkeys with the prefrontal cortex removed have a deficit in the short-term. Working memory is a form of short-term memory that integrates moment-to-moment perceptions and relates them to past experiences. PET scans and functional MRIs show that the prefrontal cortex has a diminished metabolic rate in schizophrenic patients. Research has shown that an overabundance of dopamine (D-2) receptors during the development of the striatum result in excess dopamine and is associated with the malfunction of the prefrontal cortex, features also associated with schizophrenia.
CONCLUSION AND THE FUTURE DIRECTION OF NEUROSCIENCE RESEARCH
As Kandel himself intimates the experimental methodology discussed here, including his neural analogs of learning in Aplysia and long-term potentiation in the hippocampus of mice, seem at times unnatural and artificial, both as to repeated electrical stimulation and the injection of neurotransmitters, such as glutamate and serotonin, directly into neurons and their extensions and synapses. This methodology at times seems artificial, comparable to using large (pharmacologic) doses of drugs in animals to test for mutagenic and carcinogenic properties, as opposed to observing the effect of biological and physiological amounts of substances that are usually harmless. What if these methodologies are more akin in the human brain to models of the study of neuroexcitotoxicity,[1-3] and not to physiologic learning and memory, as proposed? This proposition, improbable perhaps, needs to be considered in the study of human subjects in the future.
Kandel believes that the next problem to be solved in neurobiology is the biological nature of consciousness — how do we process information, how do we think? How do we maintain awareness of ourselves and our environment and relate them to past experience? How does an objective phenomenon such as neural activity and action potentials traveling through axons produce electrical signals that cause subjective experiences, such as pain, vision, or determining what an object is and what is used for? Different parts of the brain mediate different sensations such as touch, vision, and smell, which are then processed and integrated by the brain to produce a subjective experience. New perceptions and experiences are compared to past experiences and assessed by our emotional system to act on, to be discarded, or to be stored as a memory to be recalled at a later time (p. 382-383).
Most scientists believe that there is no specific neural correlate for the unity of consciousness, postulated to be disseminated throughout the cerebral cortex and thalamus. Francis Crick (1916–2004), the codiscoverer of DNA and breaker of the genetic code, believed that the unity of consciousness and human experience is contained in a small cluster of cells in the claustrum, a thin gray area of matter “located between the insula and extreme capsule laterally and the external capsule and putamen medially, but no solid evidence exists for this theory.” Kandel believes this would be a great area of research for years to come.
It is true that the nature and unity of consciousness, interlocked as it is with thought and inner human experience, is of paramount importance, a grand design worthy of study, biological research, and even philosophic contemplation. However, Kandel implies that all the problems have been solved and that the biological processes of human learning and memory have already been established. But have we really solved the enigmas of higher learning and complex long-term declarative memory in the human brain? I think not.
The omission of mentioning the limbic system, Papez circuit, and the known neural interconnections and clinical correlates suggest a disconnection between basic neuroscience researchers and their counterparts in clinical psychiatry, neurology, and neurosurgery.[5,6,8,9] This omission is perhaps a simple oversight. If the omission was purposeful, it implies that some basic neuroscientists are missing a golden opportunity to study and relate to their clinical colleagues, who have available the best material for the subject at hand — man and his brain. In the future it may be that as knowledge advances and the human brain necessarily becomes the ultimate target of study, basic neuroscience investigators will have to work more closely with their clinical colleagues to bridge this gap between bedside and laboratory — between mice and men — if they are to elucidate consciousness and decipher the enigma of the mind of man.
Kandel’s book is recommended for both the lay public and scholars interested in the history of neuroscience and the investigation of the mechanism of implicit learning and memory. Although Kandel does not have all the answers, his book may serve as an inspiration to young investigators in the field of neurobiology as well as clinicians, particularly in the field of neuropsychiatry.
Acknowledgment
All figures and their captions, except Figures 4 and 6, are “Reprinted from IN SEARCH OF MEMORY: THE EMERGENCE OF A NEW SCIENCE OF MIND by Eric R. Kandel. Copyright © 2006 by Eric R. Kandel. With permission of the publisher, W. W. Norton and Company, Inc. All rights reserved.”
Declaration of patient consent
Patient’s consent not required as there are no patients in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
1. Blaylock RL. Immunoexcitatory mechanisms in glioma proliferation, invasion and occasional metastasis. Surg Neurol Int 2013;4:15. Available from: https://surgicalneurologyint.com/surgicalint-articles/immunoexcitatory-mechanisms-in-glioma-proliferation-invasion-and-occasional-metastasis/ [Last accessed on 28 Jul 2020].
2. Blaylock RL, Maroon J. Immunoexcitotoxicity as a central 7. mechanism in chronic traumatic encephalopathy — A unifying hypothesis. Surg Neurol Int 2011;2:107. Available from: https://surgicalneurologyint.com/immunoexcitotoxicity-as-a-central-mechanism-in-chronic-traumatic-encephalopathy-a-unifying-hypothesis/ [Last accessed on 28 Jul 2020].
3. Blaylock RL, Maroon J. Natural plant products and extracts that reduce immunoexcitotoxicity-associated neurodegeneration and promote repair within the central nervous system. Surg Neurol Int 2012;3:19. Available from: https://surgicalneurologyint.com/surgicalint-articles/natural-plant-products-and-extracts-that-reduce-immunoexcitotoxicity-associated-neurodegeneration-and-promote-repair-within-the-central-nervous-system/ [Last accessed on 28 Jul 2020].
4. Faria MA. Religious morality (and secular humanism) in Western civilization as precursors to medical ethics: A historic perspective. Surg Neurol Int 2015;6:105. Available from: https://surgicalneurologyint.com/surgicalint-articles/religious-morality-and-secular-humanism-in-western-civilization-as-precursors-to-medical-ethics-a-historic-perspective/ [Last accessed on 28 Jul 2020].
5. Faria MA. Violence, mental illness, and the brain — a brief history of psychosurgery: Part 2-from the limbic system and cingulotomy to deep brain stimulation. Surg Neurol Int 2013;4:75. Available from: https://surgicalneurologyint.com/surgicalint-articles/violence-mental-illness-and-the-brain-a-brief-history-of-psychosurgery-part-2-from-the-limbic-system-and-cingulotomy-to-deep-brain-stimulation/ [Last accessed on 28 Jul 2020].
6. Gilman S, Newman SW. Manter and Gatz’s Essential of Clinical Neuroanatomy and Neurophysiology. 8th ed. Philadelphia, PA: FA Davis Co.; 1992, p. 216-61.
7. Kandel ER. Search of Memory: The Emergence of a New Science of Mind. New York: WW Norton; 2006.
8. Robison RA, Taghva A, Liu CY, Apuzzo ML. Surgery of the mind, mood and conscious state: An idea in evolution. World Neurosurg 2012;77:662-86.
9. Salloway S, Malloy P, Cummings JL, editors. The Neuropsychiatry of Limbic and Subcortical Disorders. Washington, DC: American Psychiatry Press; 1997. Available from: https://haciendapublishing.com/articles/book-review-neuropsychiatry-limbic-and-subcortical-disorders [Last accessed on 28 Jul 2020].
10. Szasz TS. Is mental illness a disease? The therapeutic state. In: The Freeman—Ideas on Liberty; 1999, p. 38-9.
Miguel A. Faria, M.D., is Associate Editor in Chief in socioeconomics, politics, medicine, and world affairs of Surgical Neurology International (SNI).
How to cite this article: Faria MA. The neurobiology of learning and memory — as related in the memoirs of Eric R. Kandel. Surg Neurol Int 2020;11:252. Available from: https://surgicalneurologyint.com/surgicalint-articles/the-neurobiology-of-learning-and-memory-as-related-in-the-memoirs-of-eric-r-kandel/.
Copyright ©2020 Miguel A. Faria, Jr., M.D.